Trials to begin for new treatment of Trypanosomiasis
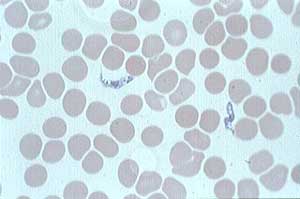 Aug. 25, 2005 (Vernon Hills, Ill.) — Immtech International, Inc. announced today that it has initiated, in partnership with the University of North Carolina at Chapel Hill, a Phase III pivotal trial of its oral drug DB289 in the Democratic Republic of Congo to treat Trypanosomiasis (African sleeping sickness) and that it is screening patients for enrollment. The study is planned at multiple sites in the Democratic Republic of Congo, New Sudan, and Angola. The Company plans to enroll approximately 250 “stage-one” patients in a randomized trial which will compare the effectiveness of DB289 to pentamidine, the current first line therapy in Africa which is difficult to administer in remote areas and is associated with significant side-effects.
Aug. 25, 2005 (Vernon Hills, Ill.) — Immtech International, Inc. announced today that it has initiated, in partnership with the University of North Carolina at Chapel Hill, a Phase III pivotal trial of its oral drug DB289 in the Democratic Republic of Congo to treat Trypanosomiasis (African sleeping sickness) and that it is screening patients for enrollment. The study is planned at multiple sites in the Democratic Republic of Congo, New Sudan, and Angola. The Company plans to enroll approximately 250 “stage-one” patients in a randomized trial which will compare the effectiveness of DB289 to pentamidine, the current first line therapy in Africa which is difficult to administer in remote areas and is associated with significant side-effects.
Human African trypanosomiasis, also known as sleeping sickness, is caused by single-celled parasites, Trypanosoma brucei, which are transmitted to humans by infected tsetse flies. The illness produces fever, lymph node inflammation, eventual impairment of the brain and nervous system in its late stage and, if not treated, death. Stage-one patients are those infected with the African sleeping sickness parasites in their blood and lymph nodes. If untreated, the parasites will enter patients’ cerebrospinal fluid and brain and cause a more severe form of the disease. The trial is not blinded because DB289 is an oral drug and pentamidine is administered as an intramuscular injection.
 One half of the patients with the confirmed disease will receive DB289 twice daily (100 mg) for ten consecutive days. The remaining control group patients will receive the standard therapy of once daily injections of pentamidine. Both patient groups will be monitored for clearance of the parasite during treatment and at specified intervals after the treatment regimen is completed. The study protocol was reviewed and approved by the United States Food & Drug Administration (U.S. FDA) as part of a Special Protocol Assessment. During the Phase III trial, the Company intends to submit to the U.S. FDA a Treatment IND for approval which, if granted, would enable DB289 to be sold for the treatment of Trypanosomiasis in desperately ill patients while the clinical trials are being completed.
One half of the patients with the confirmed disease will receive DB289 twice daily (100 mg) for ten consecutive days. The remaining control group patients will receive the standard therapy of once daily injections of pentamidine. Both patient groups will be monitored for clearance of the parasite during treatment and at specified intervals after the treatment regimen is completed. The study protocol was reviewed and approved by the United States Food & Drug Administration (U.S. FDA) as part of a Special Protocol Assessment. During the Phase III trial, the Company intends to submit to the U.S. FDA a Treatment IND for approval which, if granted, would enable DB289 to be sold for the treatment of Trypanosomiasis in desperately ill patients while the clinical trials are being completed.
T. Stephen Thompson, President and CEO, said, “DB289 is an important new oral drug that may have a major impact on a devastating disease that has, for centuries, been a scourge in the sub-Sahara. The entry of DB289 into a Phase III pivotal trial represents the accomplishment of a major milestone for both our Company and infected patients, given the significant challenges present in rural areas where the neediest patients are located. In addition to targeting against Trypanosomiasis, DB289 is in a Phase IIb trial to treat malaria, and we are planning to initiate pivotal Phase III trials in malaria treatment and Pneumocystis pneumonia in AIDS patients. We also plan to initiate clinical trials of DB289 in malaria prophylaxis.”
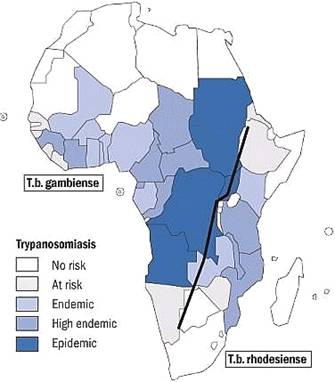
Doctors Without Borders estimates that the geographical range in sub- Sahara Africa where human African sleeping sickness occurs encompasses 36 countries, where more than 60 million people are at risk of contracting the disease. The World Health Organization (WHO) estimates that there are 300,000 to 500,000 active cases of human African sleeping sickness in central Africa. A WHO survey reports that an “epidemic situation” for African sleeping sickness exists in the sub-Sahara region of Africa, which includes the countries of Angola, Sudan, Uganda and the Democratic Republic of the Congo.
About Immtech International
Immtech International, Inc. is a pharmaceutical company advancing the development and commercialization of oral drugs to treat infectious diseases and neoplastic (cancer) and metabolic (diabetes) disorders. We are developing treatments for fungal infections, malaria, tuberculosis, cancer, diabetes, Pneumocystis pneumonia, and tropical diseases, including African sleeping sickness (trypanosomiasis) and leishmaniasis. We have a worldwide, exclusive license to commercialize a dicationic pharmaceutical platform from which a pipeline of products may be developed to target large, global markets. Our dicationic pharmaceutical platform, which includes DB289, was developed by a scientific consortium led by scientists at the University of North Carolina at Chapel Hill and Georgia State University.
“Safe Harbor” Statement under the Private Securities Reform Act of 1995: Statements in this press release regarding Immtech International, Inc.’s business which are not historical facts are “forward-looking statements” that involve risks and uncertainties. For a discussion of such risks and uncertainties that could cause actual results to differ from those contained in the forward-looking statements, see “Risk Factors” in the Company’s Annual Report on Form 10-K for the most recently ended fiscal year.
